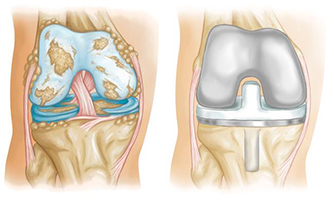
OPTAENK knee replacement
ASTM F 2083:2006: Standard Specification for Total Knee Prosthesis.
ISO 14243-1:2009: Implants for Surgery - Wear of Total Knee Joint Prostheses - Part 1: Parameters for Loading and Displacement in Wear-Testing Machines, Including Load Control and Environmental Conditions.
ISO 14879-1:2000: Implants for Surgery - Total Knee-Joint Prostheses - Part 1: Determination of Endurance Properties of Tibial Trays.
ASTM F 1800: Standard Test Method for Cyclic Fatigue Testing of Metal Tibial Tray Components in Total Knee Joint Replacements.
ASTM F 897: Standard Test Method for Measuring Fretting Corrosion in Osteosynthesis Plates and Screws for Total Knee Joint Replacements.
ASTM F 1672:1995: Standard Specification for Patellar Resurfacing Prosthesis.
ASTM F 1223:2005: Standard Test Method for Determination of Constraint in Total Knee Replacements.
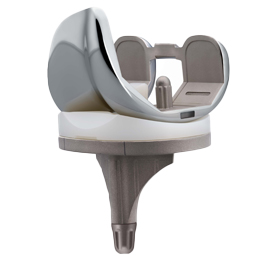
- Anatomical Trochlear Design: Optimizes patellar tracking, reduces stress on the patellar tendon, and lowers the risk of patella dislocation.
- Bone-Preserving Design: Minimizes condylar resections and offers a posterior-stabilized version without requiring a box cut.
- Flexible Tibial Extension Options: Various cemented tibial extension stems are available to provide additional stabilization when needed.
- J-Curved Sagittal Design: Enhances natural knee kinematics, improves knee flexion, and supports the rollback of the femoral component.
The anatomical design of the trochlea optimizes the patellar tracking path, significantly reducing the risk of patella dislocation by minimizing tension on the patellar tendon.
This design emphasizes maximum bone preservation, achieving posterior stability (PS) with minimal condylar resection and without the need to remove the space between the condyles.
The J-shaped sagittal profile of the prosthesis enhances the knee’s range of motion to approach a natural state, improves knee flexion, and facilitates the backward rollback of the femoral component.
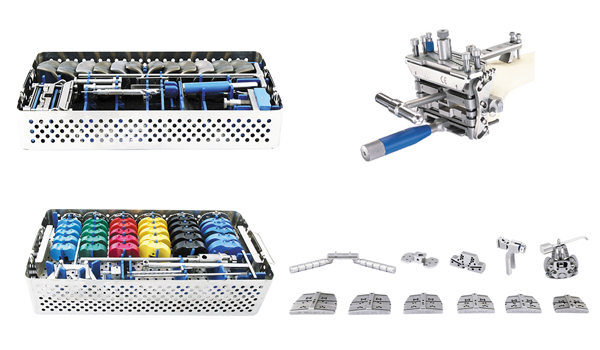
The OPTA ENK prosthesis is designed with three key features:
-
Simple and Efficient Surgical Tools: These tools are designed to enable precise, efficient, and rapid surgery, while also allowing for the performance of various techniques and tests during the procedure.
-
Optimized Implant Design and Size Range: Based on a morphometric study, the design aims to optimize the contact surfaces between bone and implant. Features include grooved side fins for enhanced strength and an internal groove in the femoral component for improved stability.
-
Interchangeable Joint Profiles: This feature allows different implant sizes to be exchanged without compromising the contact surfaces between metal and polyethylene.
The femoral component is made of Cobalt-Chrome Alloy (Co-Cr-Mo) in accordance with ISO 5832-4. It is available in 6 sizes for the left and 6 sizes for the right knee. This component comes in two designs: PS (Posterior Stabilized) and STD (Standard). Additionally, an internal groove is embedded in the femoral piece to enhance its stability.

The tibial component is made from Titanium Alloy (Ti6Al4V) in accordance with ISO 5832-3. It features a symmetrical geometry and is available in 6 sizes, suitable for both left and right legs. The design includes grooved side fins to enhance strength and stability.

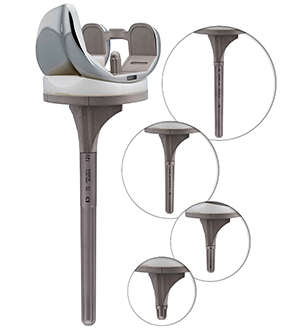
This part is made from titanium alloy and conforms to the ISO 5832-3 standard, featuring a grooved design. The extension stem, with a diameter of 12.5 mm, is available in 5 different lengths: 10 mm, 30 mm, 60 mm, 90 mm, and 120 mm.
UHMWPE GUR 1020 tibial insert with ISO 5834-2, Range: 6 sizes, thickness: 9, 11, 13, 16, 19 mm. This component is available in two different PS and STD designs.

The UHMWPE GUR 1020 resurfacing patella, compliant with ISO 5834-2, is available in four sizes: Ø 30 mm, 33 mm, 36 mm, and 39 mm. This component is installed on the back of the patella after it has been cut and removed. It is secured in place using cement and three small triangular bases positioned at equal distances from each other.

Screw component for tibial base: Titanium Alloy (Ti6Al4V) screw component for tibial base with ISO 5832-3 (Ø 6.5 mm, 6 lengths: 20, 25, 30, 35, 40, and 45).
Screw component for PS insert: Titanium Alloy (Ti6Al4V) screw component for PS insert with ISO 5832-3, (Ø 5 mm, length: 15 mm).

Evolis total knee replacement
ASTM F 2083:2006 Standard Specification for Total Knee Prosthesis.
ISO 14243-1:2009 Implants for surgery - Wear of total knee joint prostheses - Part 1:
Loading and displacement parameters for wear-testing machines with load control and corresponding environmental conditions for test.
ISO 14879-1:2000 Implants for surgery - Total knee-joint prostheses - Part 1:
Determination of endurance properties of knee tibial trays.
ASTM F 1800 Standard Test Method for Cyclic Fatigue Testing of Metal Tibial Tray Components of Total Knee Joint Replacements Constraint.
ASTM F 897 Standard Test Method for Measuring Fretting Corrosion of Osteosynthesis Plates and Screws Components of Total Knee Joint Replacements Constraint.
ASTM F 1672:1995 Standard Specification for Resurfacing Patellar Prosthesis.
ASTM F 1223:2005 Standard Test Method for Determination of Total Knee Replacement Constraint.
- Anatomical design of the trochlea optimizes the patellar tracking, reducing stress on patellar tendon and the risk of patella dislocation.
- Bone preserving design: minimum condylar resections and posterior-stabilised version without need for a box.
- Different cemented tibial extension stem options are available when further stabilization is needed.
- J-curved sagittal allows more nature knee kinematic improves knee flexion and promotes rollback of the femoral component.

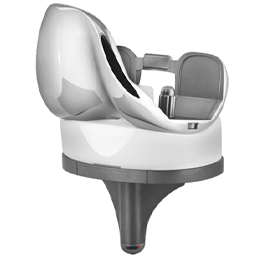
Evolis total knee replacement prosthesis is available in two designs:
- Posterior-Stabilized (PS) Design.
- Standard (STD) Design.
The Evolis concept is based on 3 main features:
- A compact, easy and well-designed ancillary which allows an accurate and fast surgical act, offering at the same time several operative choices as well as checks during surgery.
- Both implant design and product range are the result of a morphometric study aimed at the optimization of bone-implant surfaces.
- The choice of common profiles allows interchangeability of the sizes without compromising on maximal metal - polyethylene contact surfaces.
Cobalt-Chrome Alloy (Co-Cr-Mo) femoral component with ISO 5832-4, Range: 6 left sizes, 6 right sizes. This component is available in two different PS and STD designs.

Titanium Alloy (Ti6Al4V) tibial component with ISO 5832-3, Range: 6 sizes.

Titanium Alloy (Ti6Al4V) tibial extension stem with ISO 5832-3, (Ø 12.5 mm, 3 Lengths: 30, 60, 90 mm).

UHMWPE GUR 1020 tibial insert with ISO 5834-2, Range: 6 sizes, thickness: 9, 11, 13, 16, 19 mm. This component is available in two different PS and STD designs.

UHMWPE GUR 1020 resurfacing patella with ISO 5834-2, Range: (4 sizes, Ø 30, 33, 36, 39 mm).

Screw component for tibial base: Titanium Alloy (Ti6Al4V) screw component for tibial base with ISO 5832-3 (Ø 6.5 mm, 6 lengths: 20, 25, 30, 35, 40, and 45).
Screw component for PS insert: Titanium Alloy (Ti6Al4V) screw component for PS insert with ISO 5832-3, (Ø 5 mm, length: 15 mm).

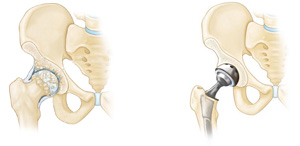
Sheikh Cementless Hip Implants

ASTM F 2009 Standard Test Method for Determining the Axial Disassembly Force of Taper Connections of Modular Prostheses.
ASTM F 1820 Standard Test Method for Determining the Forces for Disassembly of Modular Acetabular Devices.
ISO 7206-4 Implants for surgery – Partial and total hip joint prostheses - Part 4:
Determination of endurance properties and performance of stemmed femoral components.
ISO 7206-6 Implants for surgery – Partial and total hip joint prostheses - Part 6:
Endurance properties testing and performance requirements of neck region of stemmed femoral components.
The CCD angle for the stem is common at 135ᵒ for standard offset. Sheikh total hip replacement prosthesis has trapezoidal cross-sectional proximal stem design. Trapezoidal cross-section shape of stem causes to better torsional stability in femoral bone and has major effect on the stress distribution in physiological load in gait cycle and sits to stand task than other various proximal femoral stem shapes.
Titanium alloy cementless stem is fully HA coated.
Cementless acetabular cup has a central hole in the base of the shell that is used for shell insertion. This base hole has a slot to enhance torsional manipulation of the shell during insertion.
Titanium alloy cementless femoral stem in accordance with ISO 5832-3 and 12/14 taper, available in 8 sizes (9 to 16) and fully HA coated.

Titanium alloy cementless acetabular cup available in 9 sizes (48 to 64). and in 3-hole configuration (ISO 5832-3).

Stainless steel femoral head with 12/14 taper, available in external diameters of 32 and 36 mm in 4 neck sizes (S, M, L, XL) (ISO 5832-1).

The logic liner in UHMWPE GUR 1020 grade available in 4 sizes (48 to 64) (ISO 5834-2).

Screw (Ti6Al4V) in accordance with ISO 5832-3.
Sheikh Cemented Hip Implants

ASTM F 2009 Standard Test Method for Determining the Axial Disassembly Force of Taper Connections of Modular Prostheses.
ASTM F 1820 Standard Test Method for Determining the Forces for Disassembly of Modular Acetabular Devices.
ISO 7206-4 Implants for surgery – Partial and total hip joint prostheses - Part 4:
Determination of endurance properties and performance of stemmed femoral components.
ISO 7206-6 Implants for surgery – Partial and total hip joint prostheses - Part 6:
Endurance properties testing and performance requirements of neck region of stemmed femoral components.
The CCD angle for the stem is common at 135ᵒ for standard offset. Sheikh total hip replacement prosthesis has trapezoidal cross-sectional proximal stem design. Trapezoidal cross-section shape of stem causes to better torsional stability in femoral bone and has major effect on the stress distribution in physiological load in gait cycle and sits to stand task than other various proximal femoral stem shapes.
Stainless steel cemented femoral stem in accordance with ISO 5832-1 and 12/14 taper, available in 9 sizes (8 to 16).

Cemented acetabular cup in UHMWPE GUR 1020 grade available in 9 sizes (44 to 60) (ISO 5834-2).

Stainless steel (ISO 5832-1) femoral head with 12/14 taper, available in external diameter of 28mm in 5 neck sizes (S, M, L, XL, XXL).

Sheikh Bipolar Cup

ASTM F 1820 Standard Test Method for Determining the Forces for Disassembly of Modular Acetabular Devices.
- Femoral neck and trochanteric fractures of the proximal femur.
- Osteonecrosis of the femoral head.
- Revision procedures where other devices or treatments for these indications have failed.
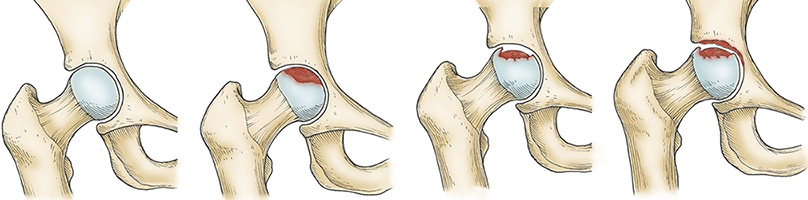
SHEIKH bipolar cup is designed to prevent serious bone destruction consequent and used as a treatment for femoral neck fractures. UHMWPE cup has a beveled margin to avoid neck cup impingement in internal and external rotation in gait cycle and physiological cyclic loading. Sheikh bipolar prosthesis in sitting and squatting movement provides patient with normal range of motion at hip.
Stainless Steel Bipolar shell (LVM 316 alloy-ISO 5832-1)
Cross-linked UHMWPE Bipolar cup

Bipolar Cup is available in 10 sizes (Ø 40 to 58 mm) with the ability to use with cementless and cemented stem.
This cup can be used with femoral head only in Ø 28 diameter with 5 neck sizes (S, M, L, XL, XXL).
Cross-linked UHMWPE Ring (ISO 5834-2)
Stainless Steel Wire (LVM 316 alloy - ISO 5832-1).


